In a groundbreaking development reported in *Internal Medicine (Tokyo, Japan)*, a medical team from St. Marianna University School of Medicine has successfully implemented a novel procedure to enable safer, more efficient plasmapheresis for a patient with severe myasthenia gravis. This advancement could signal a shift in how patients with myasthenia gravis and similar conditions requiring frequent blood purification therapies are managed.
DOI: 10.2169/internalmedicine.2990-23
A Decade of Challenges in Managing Myasthenia Gravis
A case report authored by Dr. Fumiya Kitano and colleagues detailed the treatment journey of a 41-year-old woman diagnosed with seronegative myasthenia gravis. Despite aggressive therapies, she suffered recurrent myasthenic crises roughly every three months for several years.
Vascular Access Complications
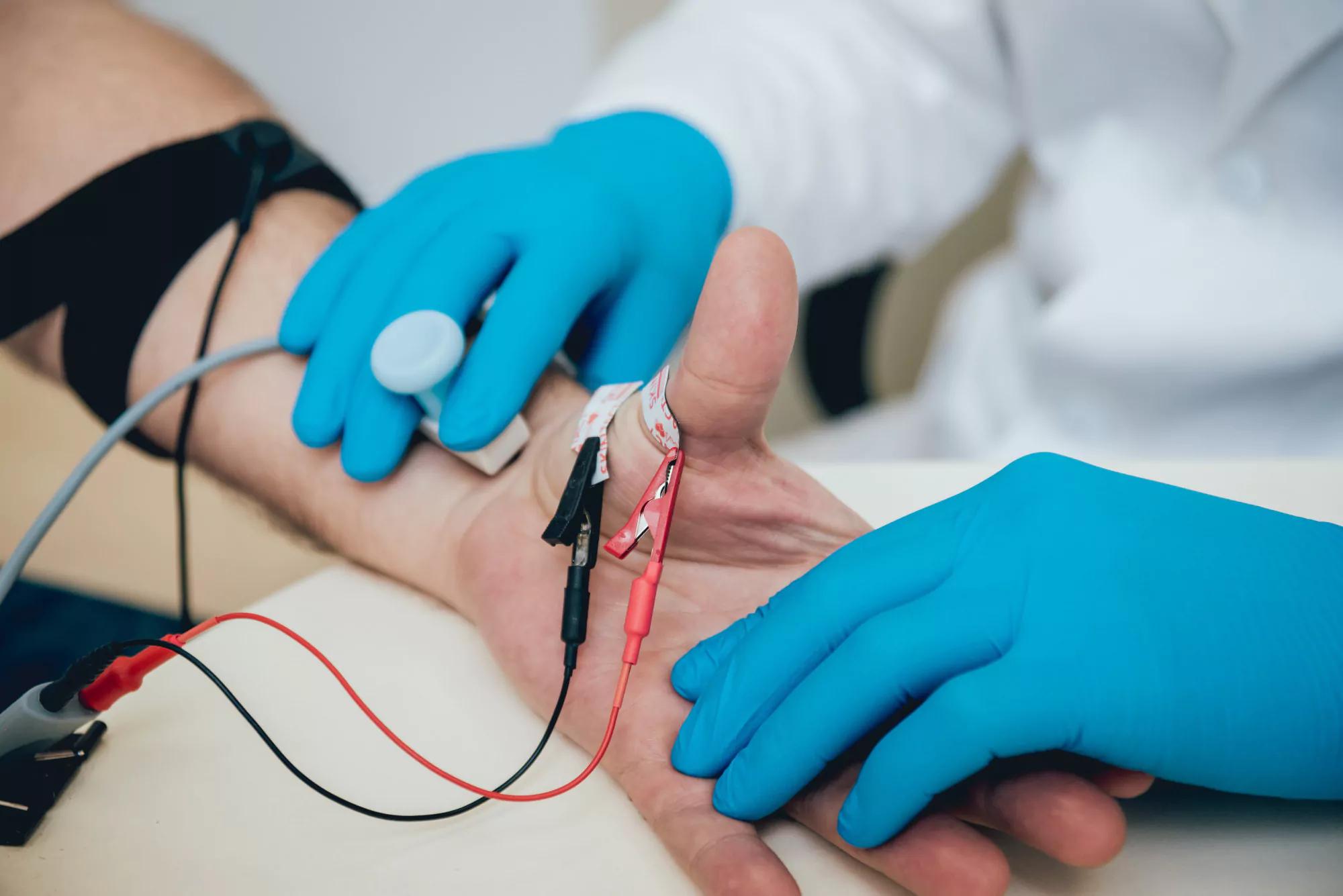
Traditional methods for administering plasmapheresis involved using a non-tunneled non-cuffed central venous dialysis catheter (NTNCC). However, the patient experienced multiple complications, such as catheter-related thrombosis, and the side effects of steroid treatment, such as significant weight gain, made catheter insertion increasingly difficult.
The Superficialized Brachial Artery: A Solution
In response to these complications and the patient’s need for regular plasmapheresis, the medical team sought a more permanent vascular access (VA) solution. Their innovative approach entailed the creation of a subcutaneously superficialized brachial artery as the VA site.
Uninterrupted Therapy and Patient Safety
This new VA technique allowed the patient to undergo uninterrupted and safer apheresis therapy. The success of this case opens the possibility of such a technique being a viable alternative for other patients with similar conditions and difficulties with traditional VA methods.
References
1. Kitano, F., Marui, Y., Sakurai, K., Shibagaki, Y., Sakurada, T., & Kojima, S. (2024). Use of the Superficialized Brachial Artery as Vascular Access for a Patient with Myasthenia Gravis with a Frequent Need for Plasmapheresis: A Case Report. Internal Medicine (Tokyo, Japan). https://doi.org/10.2169/internalmedicine.2990-23
2. Conti-Fine, B. M., Milani, M., & Kaminski, H. J. (2006). Myasthenia gravis: past, present, and future. Journal of Clinical Investigation, 116(11), 2843–2854. https://doi.org/10.1172/JCI29894
3. Howard, J. F. (2015). Clinical Overview of MG. Myasthenia Gravis Foundation of America. Retrieved from https://myasthenia.org/For-Professionals/Clinical-Overview-of-MG
4. Juel, V. C., & Massey, J. M. (2007). Myasthenia gravis. Orphanet Journal of Rare Diseases, 2, 44. https://doi.org/10.1186/1750-1172-2-44
5. Wolfe, G. I., Kaminski, H. J., Aban, I. B., et al. (2016). Randomized Trial of Thymectomy in Myasthenia Gravis. The New England Journal of Medicine, 375, 511-522. https://doi.org/10.1056/NEJMoa1602489
Keywords
1. Myasthenia Gravis Treatment
2. Plasmapheresis Vascular Access
3. Superficialized Brachial Artery
4. Permanent Vascular Access Technique
5. Blood Purification Therapy Myasthenia
Conclusions and Future Directions
The innovative use of a superficialized brachial artery for permanent vascular access presents a significant advancement in the management of patients requiring regular blood purification therapies. As evidenced by this case report, innovative medical techniques can improve the quality of life and treatment outcomes for individuals with chronic illnesses such as myasthenia gravis.
The medical community eagerly anticipates further developments. More comprehensive studies could cement the superficialized brachial artery technique as a standard of care, offering hope to many struggling with the challenges of frequent plasmapheresis.